Bond dissociation enthalpy of E—H (E = element) bonds is given below. Which of the compounds will act as strongest reducing agent?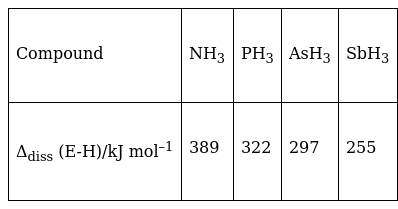
A reducing agent is a compound or element that losses electron.
A strong reducing agent looses electron more easily as compared to weak reducing agent.
SbH3 acts as strongest reducing agent due to minimum value of bond dissociation enthalpy.
It requires less bond dissociation enthalpy to break bonds, therefore it can easily donate electrons to other chemical species.
NOTE: lesser will be bond dissociation enthalpy, less time is taken to break the bond between atom and more easily it can loses electrons to form bond with another atom
Therefore stronger is the reducing agent.
Hence the correct option is (iv)
1