Which of the following acids forms three series of salts?
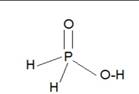
H3PO2 is monobasic as it consist of only one OH group.
Structure of H3PO2:
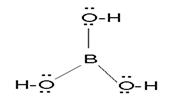
H3BO3: it does not act as proton donor but behaves as lewis acid. Boric acid does not dissociate in aqueous solution. It can be predicted from its structure that it have 3 OH group. Boric acid first accept one OH- then it liberates one electron per molecule. Therefore it is monobasic.
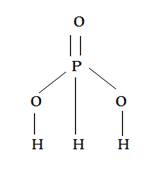
H3PO3: it is dibasic as it have two OH group.
structure of H3PO4:
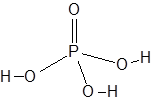
It has three OH group therefore it is tri basic. Oxidation state of phosphorous in H3PO4 is +5.
As it has three OH group, it means that it has 3 ionisable hydrogen atoms and hence form three series of salts. These are:
1.NaH2PO4
2. NaHPO4
3. Na3PO4
Hence the correct option is (iii).