Strong reducing behavior of H3PO2 is due to
All oxyacids of phosphorous which have P-H bond acts as strong reducing agent. H3PO2 has two P-H bond. Therefore it acts as strong reducing agent.
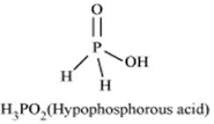
Hence due to presence of one O-H bond and two P-H bond H3PO2 is strong reducing agent. Hence the correct option is (iii).
1