In solid state PCl5 is a _________.
in solid state, PCl5 exists as ionic solid [PCl4]+[PCl6]- in which cation is tetrahedral and anion is octahedral.
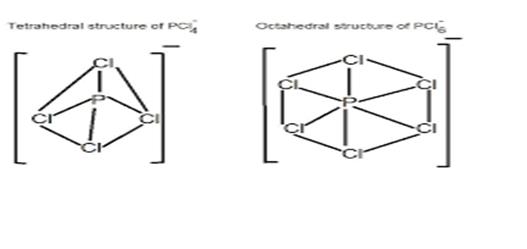
Hence the correct option is (iv)
2
In solid state PCl5 is a _________.
in solid state, PCl5 exists as ionic solid [PCl4]+[PCl6]- in which cation is tetrahedral and anion is octahedral.

Hence the correct option is (iv)