Assertion : ![]() for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason : For weak electrolytes degree of dissociation increases with dilution of solution.
(i) Both assertion and reason are true and the reason is the correct explanation of assertion.
Λmfor weak electrolytes shows a sharp increase when the electrolytic solution is diluted as degree of dissociation and the number of ions in total volume of solution that contains 1 mol of electrolyte increases with dilution of solution.
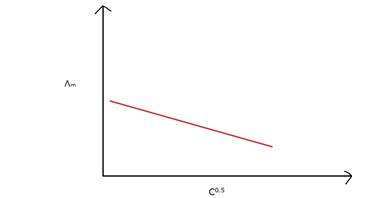
Where, C= Concentration
Hence, Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted because degree of dissociation increases with dilution of solution.
1