Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.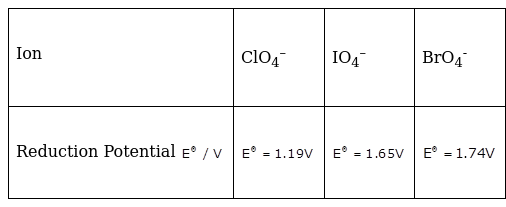
REDUCTION POTENIAL: reduction potential is a measure of the ease with which a molecule will accept electrons.
OXIDISING POWER: it is the ability to gain electrons.
Therefore oxidizing power is directly proportional to reduction potential.
Greater is the reduction potential, more will be its oxidizing power.
Hence the order is BrO4–> IO4–>ClO4
Thus the correct option is (iii).
1