Match items of Column I with the items of Column II and assign the correct code :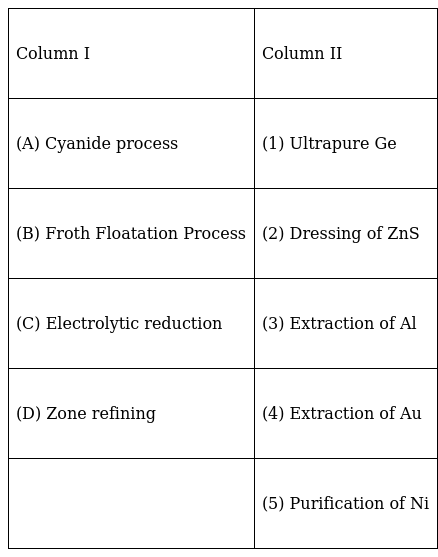
Code :
A. (4) For the extraction of Au, cyanide process is used. An anionic complex [Au(CN)2]- forms which is reduced by Zn to pure Au.
4Au(s) + 8CN– (aq) + 2H2O(aq) + O2(g) → 4[Au(CN)2] – (aq) + 4OH– (aq)
2[Au(CN)2] – (aq) + Zn(s) → 2Au(s) + [Zn(CN)4] 2– (aq)
B. (2) By adjusting the proportional of oil to water or by adding depressants in Froth floatation method, an ore can be selectively separated as froth from another. If we want PbS to come out as froth but not ZnS then by adding NaCN, ZnS can be separated from PbS. This is called dressing of an ore.
C. (3) Electrolysis of Al2O3 is used to extract Aluminium. This process is called as Hall-Heroult’s process. The following reactions take place at the cathode and anode during electrolysis
Cathode: Al3+ (melt) + 3e–→ Al(l)
Anode: C(s) + O2– (melt) → CO(g) + 2e–
C(s) + 2O2– (melt) → CO2 (g) + 4e–
D. (1) Germanium is purified using zone refining method where a molten zone passes over the solid rod. The principle behind this method is that impurities are more soluble in the molten metal than in the solid metal. The pure metal stays behind while the impurities move along with the rod to an adjacent molten zone which can be eventually cut off. In the end, we get ultrapure Germanium which can be used as a semiconductor.