Consider Fig. 3.2 and answer the questions (i) to (vi) given below.
(i) Redraw the diagram to show the direction of electron flow.
(ii) Is silver plate the anode or cathode?
(iii) What will happen if salt bridge is removed?
(iv) When will the cell stop functioning?
(v) How will concentration of Zn2+ ions and Ag+ ions be affected when the cell functions?
(vi) How will the concentration of Zn2+ ions and Ag+ ions be affected after the cell becomes ‘dead’?
(i) Here, the electrons will move from Zn to Ag.
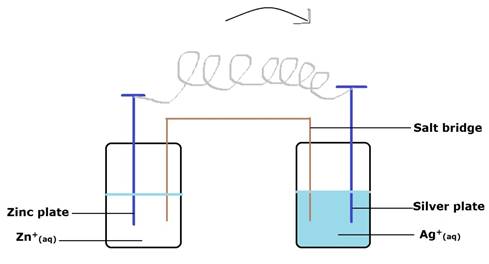
(ii) The silver plate is Cathode, the one on the right hand side (RHS).
(iii) Salt bridge maintains the electrical neutrality and connects the two half-cells.
If the salt bridge is removed, the cell will stop working as electrons will not be able to flow from one side to another. Radox reaction will not occur.
(iv) The cell will stop functioning when equilibrium is attained. In other words, When Ecell = 0
Or, Ecathode = Eanode
(v) When cell is functioning, the concentration of Zn2+ions will increase and concentration of Ag+ ions will decrease because of the radox reaction between these two chemical species.
(vi) No change in the concentration of Zn2+ and Ag+ as the cell is dead, i.e., Ecell = 0.
According to the Nernst equation,
Ecell = ![]() -
- ![]() log
log ![]()
Therefore, ![]() =
= ![]() log
log ![]() (Ecell = 0)
(Ecell = 0)
We know, ![]() value is fixed at a fixed temperature, thus, the concentration of Zn2+ and Ag+ remains the same.
value is fixed at a fixed temperature, thus, the concentration of Zn2+ and Ag+ remains the same.