Which of the following statements are correct?
(i) S–S bond is present in H2S2O6.
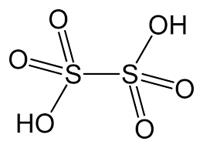
Therefore it can be clearly seen from the figure that there a bond between S-S.
(ii) In peroxosulphuric acid (H2SO5) sulphur is in +6 oxidation state.
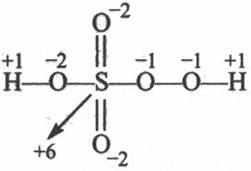
Therefore the oxidation of S is +6 . to calculate the oxidation state of S. first consider it x
+1 + (-2) + x + (-2) + (-2) + (-1 + (-1) + 1 = 0
X = 6
(iii) In Haber’s process Fe and Mo is used as catalyst
(iv) Change in enthalpy is negative for the preparation of SO3 by catalytic oxidation. The creation of sulphur dioxide is exothermic reaction.
Hence the correct options are (i) and (ii)
1