Match the formulas of oxides given in Column I with the type of oxide given in Column II and mark the correct option.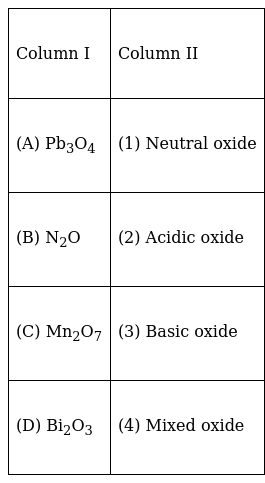
Code :
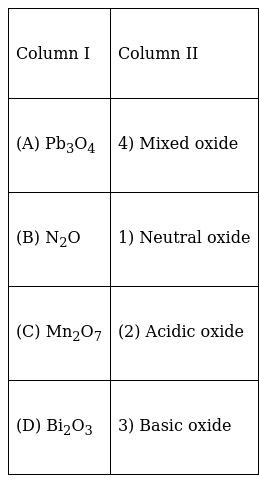
Mn2O7 on dissolution in water produce acidic oxide
Bi2O3 on dissolution in water produce basic oxide
Pb3O4 is mixed oxide of PbO.Pb2O3
N2O is neutral oxide, do not have any charge
1