Match the properties given in Column I with the metals given in Column II.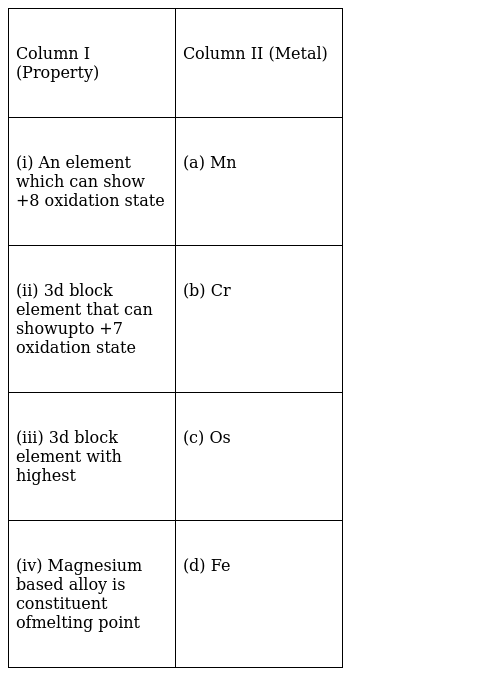
(i)-(c), (ii)- (a), (iii)-(b)
(i)-(c) Osmium has the highest oxidation state of +8 because it can expand it’s octet and use all its 8 electrons.
Electronic configuration of Os- [Xe] 4f14 5d6 6s2
5d6 and 6s2 can be excited to get an oxidation state of +8
(ii)-(a) Manganese can show upto +7 oxidation state
(iii)-(b) 3d block element with highest is Cr.
1