Match the statements given in Column I with the oxidation states given in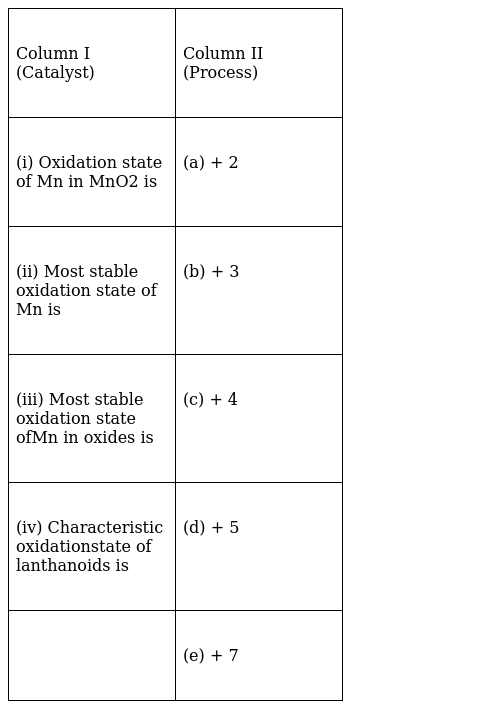
(i)-(c),(ii)-(a),(iii)-(e),(iv)-(b)
(i)-(c)- Oxidation state of Manganese in MnO2 is
x-4=0
x=+4
(ii)-(a) Lower oxidation state of Mn is very stable so +2 is stable
(iii)-(e)In KMnO4, the oxidation state of Mn is +7
(iv)-(b) Lanthanoid have 5d1 and 6s2 outermost configuration. They can lose these three electrons easily and thereby show an oxidation state of +3
1