Match the solutions given in Column I and the colours given in Column II.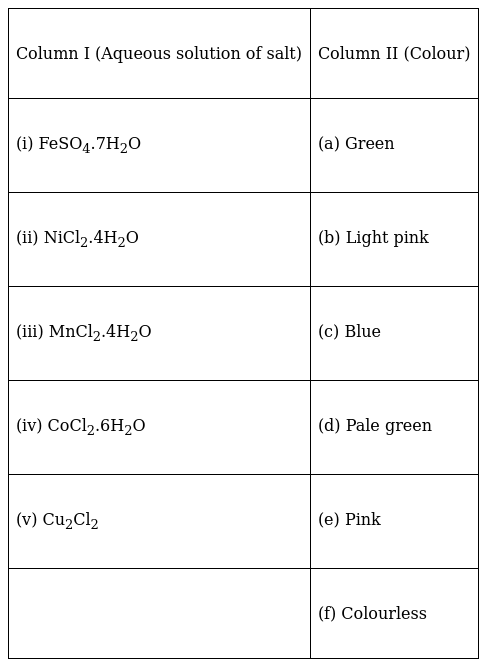
(i)-(d), (ii)-(a), (iii)-(b), (iv)-(e), (v)-(f)
(i)-(d)FeSO4.7H2O, Fe2+ has 3d6 electronic configuration. The complementary colour is pale green
(ii)-(a) NiCl2.4H2O ,Ni2+ has 3d8 electronic configuration. The complementary colour is green
(iii)-(b)MnCl2.4H2O , Mn2+ has 3d5 electronic configuration. The complementary colour is light pink
(iv)-(e)CoCl2.6H2O, Co2+ has 3d7 electronic configuration. The complementary colour is pink
(v)-(f) Cu2Cl2is colourless, Cu+ has 3d10 electronic configuration. It has a fully filled d orbital without no unpaired electrons to get excited and absorb visible light. So, Cu2Cl2 is colourless.
1