Match the property given in Column I with the element given in Column II.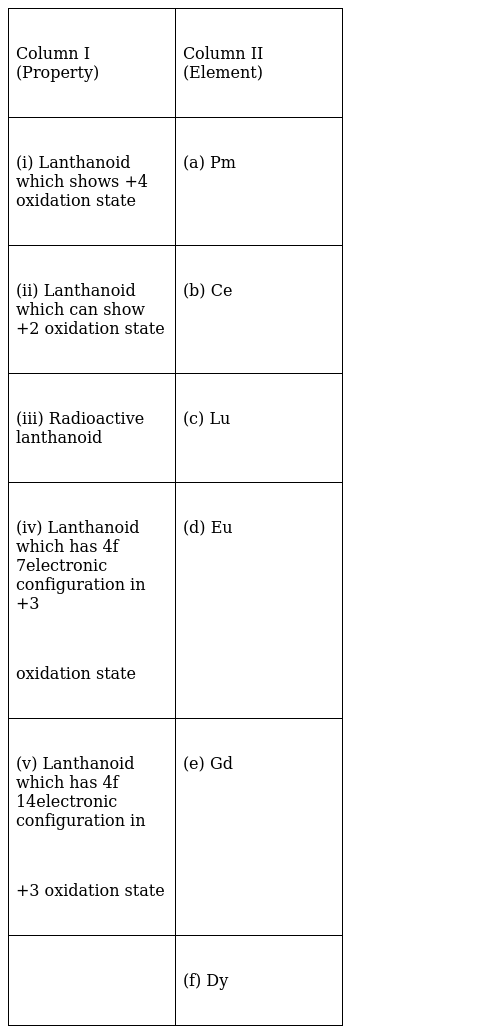
(i)-(b), (ii)-(d),(iii)-(a),(iv)-(e),(v)- (c)
(i)-(b) Ce- [Xe]4f1 5d1 6s2. Cerium can lose all 4 electrons so as to attain noble gas configuration.So, oxidation state of Ce is +4
(ii)-(d) Eu- [Xe]4f7 5d0 6s2.Europium can show +2 and +3 oxidation states.
(iii)-(a) Promethium is a radioactive lanthanoid.
(iv)-(e)Gd-[Xe]4f7 5d1 6s2. Gadolinium shows oxidation state of +3.
(v) –(c) Lu-[Xe]4f14 5d1 6s2. Lutetium has a 4f14 electronic configuration and a +3 oxidation state.
1