Match the properties given in Column I with the metals given in Column II.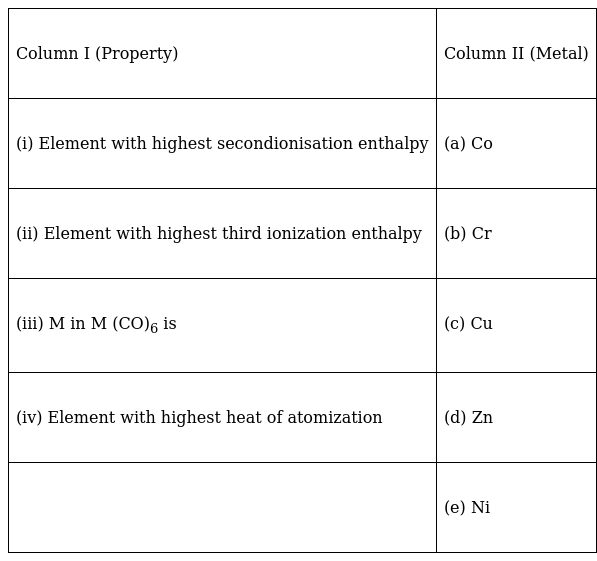
(i)-(c), (ii)-(d), (iii)-(b), (iv)-(e)
(i)-(c) Cu+ has fully filled 3d orbital with 10 electrons. Removal of one more electron is not easy and will require very high ionization energy because removing that electron disturbs the stable configuration.
(ii)-(d) For Zn2+, the electronic configuration has fully filled d orbital with 10 electrons. So, removal of one more electron is very difficult. Therefore, third ionization enthalpy is higher.
(iii)-(b) Cr forms Cr(CO)6
(iv)-(e) Nickel has the highest heat of atomization.
1