On heating lead (II) nitrate gives a brown gas “A”. The gas “A” on cooling changes to colourless solid “B”. Solid “B” on heating with NO changes to a blue solid ‘C’. Identify ‘A’, ‘B’ and ‘C’ and also write reactions involved and draw the structures of ‘B’ and ‘C’.
A is NO2 (Nitrogen dioxide)
B is N2O4 (solid colourless)
C is N2O3 (dinitrogen trioxide)
On heating lead nitrate(II) a brown gas A is produced
2Pb(NO3)2![]() 2PbO + 4NO2 + O2
2PbO + 4NO2 + O2
2NO2![]() N2O4
N2O4
2NO + N2O4![]() 2N2O3
2N2O3
STRUCTURE OF N2O4
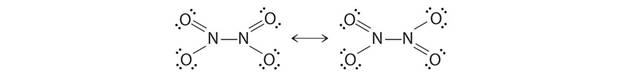
STRUCTURE OF N2O3

1