Which of the following is an amorphous solid?
• Quartz glass is an amorphous or non-crystalline solid because it lacks in a long-range order i.e. a regular long pattern of constituent particles in three dimensions which periodically repeats itself throughout the whole crystal and have a definite geometrical shape which is the case for other 3 options (i) Graphite (C), (iii) Chrome alum and (iv) Silicon carbide (SiC).
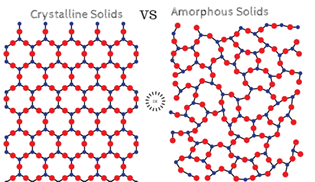
• Instead of that quartz glass has a short-range order i.e. the repetitive pattern falls through a short distance only and the regular pattern is scattered and they are disarrayed in nature (resemblances that of a liquid) unlike quartz itself which is a crystalline solid.
3