Which of the following is not the characteristic of ionic solids?
• Although the component ions of the ionic solids are not free for movement in the solid-state as they are held together by strong electrostatic forces ; they become free to move about in the molten state or aqueous state (when they get dissolved in water because) they can overcome coulombic attraction in that state and hence they have a certain amount of electrical conductivity which is high in the molten state.
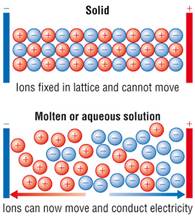
• Other 3 options (ii) Brittle nature (iii) Very strong forces of interactions and (iv) Anisotropic nature are 3 important characteristics of ionic solids
2