Graphite is a good conductor of electricity due to the presence of __________.
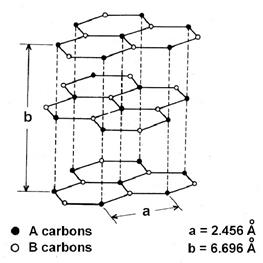
• Graphite has an exceptional but typical structure which leads to conductance.
• In this structure, the carbon atoms are arranged in multiple layers and each C atom is covalently bonded to another 3 adjacent C atoms residing in the same layer.
• And the 4th valence electrons which were left out of the C atoms are present among different layers and are therefore free for movement. These free electrons result in graphite in a good conductor.
The other 3 options, (i) lone pair of electrons (iii) cations and (iv) anions are not possible for neutral carbon atoms present in graphite.
1