Which of the following oxides shows electrical properties like metals?
• CrO2 behaves like a metal also can conduct electricity. Because Cr is a transition metal and has d-electrons (electrons present in d-orbitals) which are partially filled and leads to electrical conductivity just like metals having free electrons.
(i) SiO2 (quartz) is a network solid which is covalent in nature and always behave as an insulator ( no free electrons to conduct electricity).
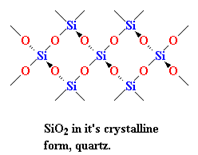
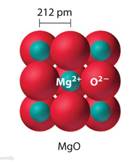
And (ii) MgO is an ionic crystalline solid which means it acts as an insulator in the solid-state( as there is no free movement for ions) and conducts electricity in the molten or aqueous state(ions overcome electrostatic attractions and are free to move).
(iii) SO2(s) is a polar molecular crystal and therefore is formed by stronger dipole-dipole interactions which make them non-conductors of electricity, unlike metals.