Cations are present in the interstitial sites in __________.
• In Frenkel defect which is also known as dislocation defect, the smaller ions (usually the cations) get dislocated from its normal lattice site to an interstitial site, therefore resulting a vacancy defect in the normal site (vacancy for cation) and simultaneously an interstitial defect in the new location of the cation.
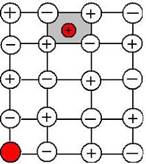
• On the other hand (ii) Schottky defect (iii) Vacancy defect (iv) Metal deficiency defect do not necessarily deal with cations.
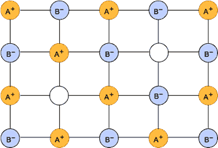
• In (ii) Schottky defect the number of missing cations and anions are equal in order to maintain electrical neutrality in the crystal and thus decreases density.
• 
• (iii)Vacancy defect occurs in case of any kind of vacancy in the crystal.
• And last (iv) Metal deficiency defect is not even a stoichiometric defect like the other options given. It is rather a non- stoichiometric defect that deals with less amount of metal as compared to the ideal stoichiometric proportion (loss of positive charge).