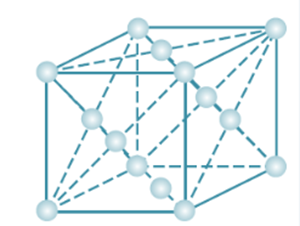
The total number of tetrahedral voids in the face centred unit cell is __________.
• We know that ,if n be the number of atoms in a crystal,
then the number of tetrahedral voids must be 2n.
• And for a face centred unit cell -
(i)there are 8 Corner atoms i.e. (8 X1/8) atoms per unit cell=1atom and
B. face-centred atom number is 6 i.e.( 6 X 1/2) atoms per unit cell= 3 atom (because each atom at the face centre is used by to adjacent unit cells)
Hence no. Of tetrahedral voids for face centred unit cell = 2(4) = 8.
Hence other 3 options (i) , (iii) and (iv) cannot be correct.
1