Which of the following point defects are shown by AgBr(s) crystals?
(A) Schottky defect
(B) Frenkel defect
(C) Metal excess defect
(D) Metal deficiency defect
• AgBr(s) has CCP(cubic close-packed) type crystals and shows both of the Schottky defect and the Frenkel defect.
• this is mainly due to the intermediate sizes of the radius of Ag+ and Br- ions for which they are able to show defect with cation dislocation in the lattice (Frankel ) and the missing of equal no.s of cations and anions (Schottky) as well.
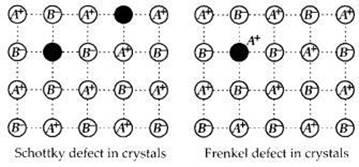
• AgBr(s) crystals do not show (C) Metal excess defect or (D) Metal deficiency defect hence other options (ii) (C) and (D) (iii) (A) and (C) (iv) (B) and (D) are all incorrect.
2