In which pair most efficient packing is present?
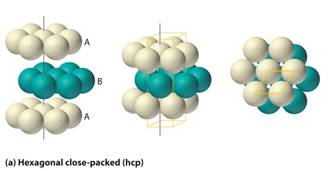
• In hcp (hexagonal close packing), the third layer and the first layer resembles each other in arrangement of the spheres and is able to cover all the tetrahedral voids.
• Since the pattern of spheres is repetitive in alternative layers, the stacking for hcp may be described as "A-B-A-B-A-B."
• The atoms in a hexagonal closest packed structure efficiently occupy 74% of space while 26% is empty space which is one of the efficient packings.
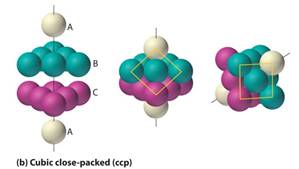
• The arrangement of ccp (cubic close packing )also efficiently fills up 74% of space( Similar to hexagonal closest packing).
• In ccp the 2nd layer of the spheres is placed on half of the depressions of the first layer and the third layer is completely different than that of the first two layers and is stacked in the depressions of the second layer, thus it covers all of the octahedral voids.
• The spheres in the third layer are not in line with those in layer 1, and the pattern of spheres does not repeat until a fourth layer is added. The fourth layer is the same as the first layer, so the arrangement of layers is "A-B-C-A-B-C."