The correct order of the packing efficiency in different types of unit cells is ________.
Packing efficiency is defined as the total space occupied by the constituent particles in a unit cell of a crystal lattice. Packing efficiency of each unit cell can be calculated.
For fcc,
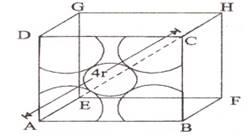
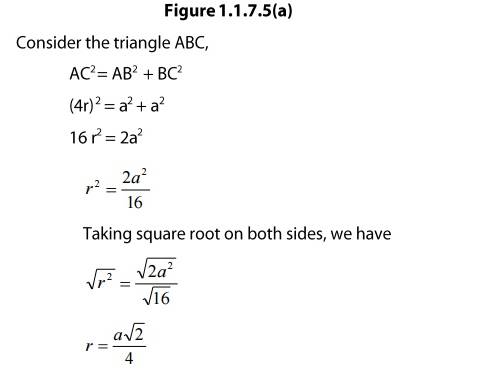
Let us consider an fcc unit cell cube with edge length a and the face diagonal which connects the centers of the particles at the edges and the face AC = b.
In ΔABC,
AC2 = b2 = BC2 + AB2
= a2 + a2 = 2a2
b = ![]() a
a
If r is the radius of the sphere, then b = 4r = ![]()
Or, ![]()
Each unit cell in in ccp has 4 spheres. Volume of each sphere is ![]() 3. So, volume of 4 spheres is 4 ×
3. So, volume of 4 spheres is 4 × ![]() 3 and the volume of the cube is a3 or [
3 and the volume of the cube is a3 or [![]() 3.
3.
Packing efficiency is then calculated as
Packing efficiency = ![]()
Substituting values, we get, P.E. =
Now we calculate the packing efficiency of bcc.
In body-centered cubic cell, the atoms are placed at the corners of the cube with one atom in the center of the cube. The central atom will be in touch with the other two diagonally arranged atoms.
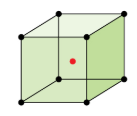
In ΔEFD, FD2 = EF2 + ED2
EF = ED = a, which is the side of the cube. FD = b.
b = ![]() a
a
In ΔAFD, let the diagonal AF = c.
c2 = a2 + b2 = a2 + 2a2 = 3a2.
c = ![]() a.
a.
The length of the diagonal of the cube is equal to 4 times of the radius of the sphere as the three spheres are along the diagonal.
Therefore, ![]() a = 4r
a = 4r
a = ![]() and r =
and r = ![]() a.
a.
This structure contains two spherical atoms and the volume is calculated as 2 × ![]() 3
3
The volume of the cube is a3. Substituting a gives [![]() ]3.
]3.
Calculating packing efficiency,
Packing efficiency = 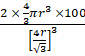 = 68%
= 68%
Now we calculate the packing efficiency of simple cubic cell.
In this arrangement, the atoms are located only at the corners of the cube and the edges of the spheres touch each other. The side of the cube, a, is equal to twice the radii r of the sphere due to this structure. This can be written as a = 2r.
Volume of the cube is (side)3 i.e. a3 = 8r3
A simple cubic cell contains only one atom, so the volume will be ![]()
Packing efficiency is calculated as
Packing efficiency = ![]()
= ![]() = 52.4%.
= 52.4%.
From these results, it is concluded that the correct answer is (ii).