Why does white ZnO (s) becomes yellow upon heating?
When zinc oxide crystals are heated or flamed with a blowtorch in the presence of air, it becomes yellow coloured on heating, and on removal of heat, goes back to white colour. This happens due to the phenomenon of metal excess defect due to presence of extra cations at interstitial sites, which is a non-stoichiometric defect. On heating ZnO, oxygen leaves as 2O2 leaving behind Zn2+ and 2 electrons. The formula is given below:
![]()
Zn2+ and the 2 electrons move to the interstitial sites of the crystal which provides excess electrons in the crystal lattice of ZnO. Also there is excess of zinc in the crystal and its formula becomes Zn1+xO. The excess Zn2+ ions move to interstitial sites and the electrons to neighbouring interstitial sites. When light falls on these crystals, these electrons absorb a part of the light in the visible region and hence impart a yellow colour to the ZnO.
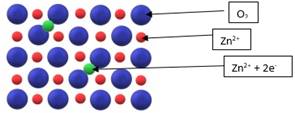
Figure 3: Metal Excess Defect due to excess cations at interstitial sites, shown in ZnO crystal. The blue spheres are O_2, the red spheres are Zn^2+, the green spheres are the excess Zn ions and electrons.