Why does the electrical conductivity of semiconductors increase with rise in temperature?
Increasing the temperature of a semiconductor causes the free electron to get more energy and crosses the energy gap to the conduction band from the valence band, as the gap between the valence band and conduction band is small. Band gap energy between the conduction and valence bands is smaller compared to insulators, which allows for electrons to be excited across the band gap, allowing for conductivity. As for comparison with metals, they would decrease in conductivity as temperature increases and a semiconductor would increase in conductivity as temperature increases. Hence, electrical conductivity of semiconductors increase with rise in temperature.
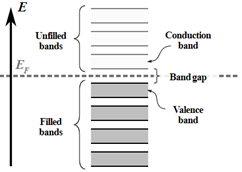
Figure 4: Simple diagram of semiconductor band structure, showing a few bands on either side of the band gap. Image obtained from Wikipedia, used under Creative Commons license: https://creativecommons.org/licenses/by-sa/4.0/deed.en