Explain why does conductivity of germanium crystals increase on doping with galium.
Germanium, which is a group 14 element, can be doped with a group 13 element like Gallium, which contains only three valence electrons. The missing fourth valence electron is called electron hole or electron vacancy. An electron from a neighbouring atom can come and fill the hole, but in doing so it would leave an electron hole at its original position. In this case, it would appear as if the electron hole has moved in the direction opposite to that of the electron that filled it. Under the influence of electric field, electrons would move towards the positively charged plate through electronic holes, while it appears as if electron holes are positively charged and are moving towards negatively charged plate. These type of semiconductors are called p-type semiconductors and this is the mechanism to increase the conductivity of germanium with doping.
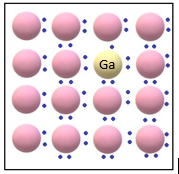
Figure 5: Doping of Germanium crystal with trivalent Gallium impurity, causing formation of electron hole, leading to p-type semiconductor.