Match the type of unit cell given in Column I with the features given in Column II.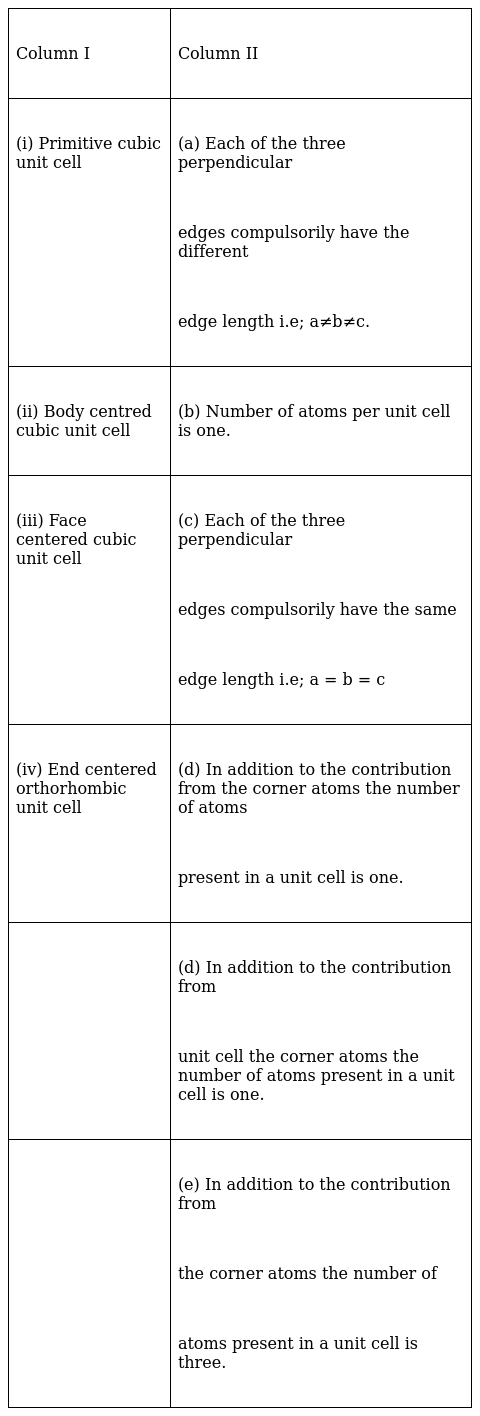
(i)-(b), (c) ,(ii)-(c),(d) , (iii)-(c), (e) , (iv)-(a), (d)
(i): for primitive cubic unit cell, a=b=c and it has one atom in each corner. So 1/8th atoms contributes in each cell. So, from all 8 corners ![]() =1.
=1.
(ii): for body centered cubic cell, a=b=c and being a body centered cell it has one atom in each corner and 1 atom in the body center. So contribution from all corners is ![]() and 1 atoms from body center atom.
and 1 atoms from body center atom.
(iii): for face centered cubic cell, a=b=c and it has one atom in each corner and 1 atom in each body face which is shared among 2 cells so contribution from one face atom is 1/2. Net contribution from all face atoms is ![]() =3. Total atoms in face centred is therefore 3+1= 4 atoms.
=3. Total atoms in face centred is therefore 3+1= 4 atoms.
(iv): In end centered orthorhombic unit cell a≠b≠c since all sides are not equal.
Total contribution of atoms present at the corners of the cubic cell = ![]()
Total contribution of atoms present at end center of the cubic cell = ![]() .
.