Match the types of defect given in Column I with the statement given in Column II.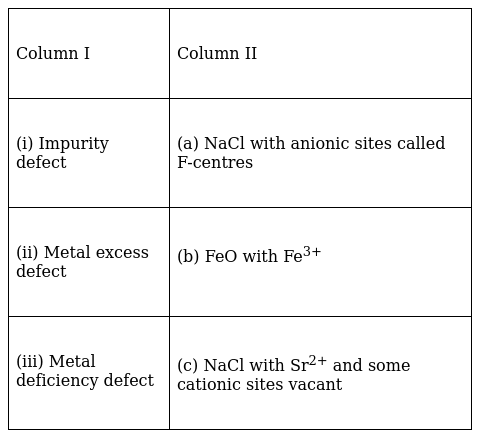
(i)-c , (ii)-a , (iii)-b
(i) Impurity Defect: This type of defect arises in crystal lattice due to presence of impurity or foreign particles in lattice. When Sr2+ is added to NaCl, Sr2+ removes to Na+ ions and occupy one site and leaves other vacant. Thus this cause cation vacancy.
(ii) Metal excess defect: When NaCl is heated in atmosphere of sodium vapour, sodium gets deposited on the surface of lattice. The inside Cl- diffuses to combine with outer sodium ion and thus combines with it with release of an electron by sodium. As a result crystal has now excess of sodium ions and the sites occupied by unpaired electron is called F centers.
(iii) Metal Deficiency defect: It is caused due to cation vacancy created by replacement of some valent ions by its higher charge ions. Example FeO with Fe3+. Where Fe2+ and Fe3+ both are present in lattice.