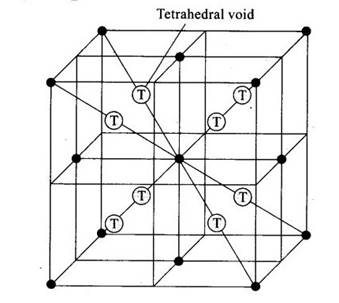
Show that in a cubic close packed structure, eight tetrahedral voids are present per unit cell.
In cubic close packing, each cube consists of eight small cubic components as shown in figure. Since atoms are present in corners and face centres, total atoms are:
Total atoms= atoms at face centres and atoms at corners
=![]() =1+3 = 4
=1+3 = 4
Total number of atoms = 4.
Considering single small cubic unit, tetrahedral void in present in body centre. Since here we have cubic structure of 8 small cubic unit, total tetrahedral voids = 8× 1 = 8.
Lets consider single cubic lattice consists of 8 small cubic units, 2 tetrahedral voids are present at each diagonal each equal distance apart from corner. There are 4 diagonal in lattice so total number of tetrahedral voids = 4× 2 =8.
Hence proved. There are 8 tetrahedral voids in CCP.