The order of reactivity of following alcohols with halogen acids is ___________.
(A) CH3CH2 —CH2—OH
(B) 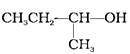
(C) 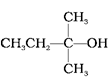
Haloalkanes are prepared from alcohols and halogen acids where the hydroxyl group of the alcohol is replaced by the halogen. Options (A) (B) and (C) are primary, secondary, tertiary alcohols respectively. Tertiary alcohols are more reactive than secondary and primary alcohol, and they form haloalkanes from haloacids at room temperature without catalysts. The order of reactivity of alcohols is 3°>2°>1°. Hence, the correct option is (ii).
4