Arrange the following compounds in the increasing order of their densities.
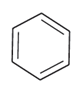
(a)
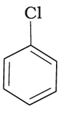
(b)
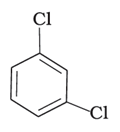
(c)
(d)
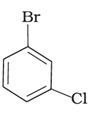
Alkyl halides are heavier in density than water. Their densities are based on the masses of the halogen atoms and the number of halogen atoms, and also the carbon atoms. Simply put, the atomic mass of Br is 79 and that of Cl is 35. From the molecules, (d) will have the heaviest mass, followed by (c), (b), and (a). Density is directly proportional to mass, hence the order will be the same in terms of reducing densities. The correct option is (i).
2