Arrange the following compounds in increasing order of their boiling points.
(a)
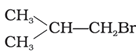
(b) CH3CH2CH2CH2Br
(c)
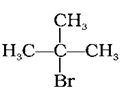
The boiling points of isomeric alkyl halides are proportional to their branching, with a decrease in B.P. with increase in branching. Hence, boiling point of the tertiary isomer is the lowest, and that of primary isomer is the highest. The order of reducing boiling points is thus (b) > (a) > (c). The correct option is (iii).
1