The reaction of toluene with chlorine in the presence of iron and in the absence of light yields ____________.
A.
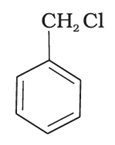
B.
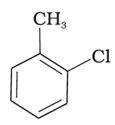
C.
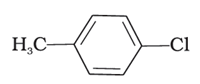
D. Mixture of (ii) and (iii)
Reaction of aromatic arenes with chlorine in the presence of Lewis acid catalysts like iron (III) chloride gives ortho and para isomers of haloarenes by electrophilic substitution reaction. Both (ii) and (iii) are products in the reaction. Cl2 forms a coordination complex with FeCl3, forming Cl+FeCl4- complex, which gives a slight positive charge to Cl and FeCl4- is negatively charged. This Cl+ then reacts with the aromatic double bonds of the toluene molecule to form an addition product, followed by deprotonation to form a mixture of o- p- and m- isomers of the chlorotoluene. The m- isomer is very unstable, so the product is not available as o- and p- are.