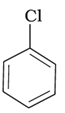
(a)
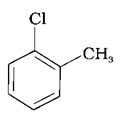
(b)
(c)
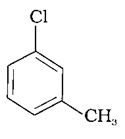
The –CH3 group is an electron releasing group, thus reducing the reactivity of aryl halides when present at ortho and para positions. Hence the aryl halides without the presence of electron releasing groups are more reactive. Hence the order of reactivity is (a) > (c) > (b). The correct option is (iv).
1