Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
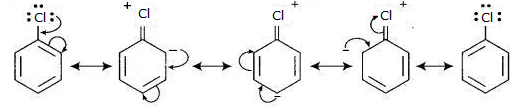
The major reason haloarenes are less reactive than haloalkanes and haloalkenes is the resonance stabilization of the aryl ring. For example, in C6H5-Cl, the electron pairs on halogen atom are in conjugation with ϖ-electrons of the ring. Due to resonance, the C—Cl bond acquires a partial double bond character, making it less reactive to nucleophilic substitution than haloalkanes and haloalkanes.
1