Discuss the role of Lewis acids in the preparation of aryl bromides and chlorides in the dark.
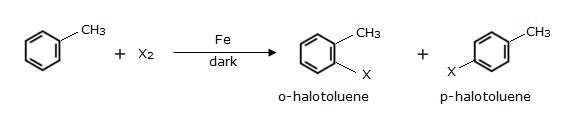
Aryl bromides and chlorides can be prepared from arenes by electrophilic substitution. This reaction is carried out by treating the arene with chlorine or bromine in the presence of iron (III) chloride in the absence of light. Iron (III) chloride, that is, FeCl3 is a Lewis acid, which generates the electrophile required to take the reaction forward. FeCl3 forms a coordination compound with Cl2, making the complex Cl+[FeCl4-]. The chloride ion gains a partial positive charge, acting as an electrophile, and attacking the ϖ bonds. The final products are o-arylhalide and p-arylhalide.
1