Which of the following compounds (a) and (b) will not react with a mixture of NaBr and H2SO4. Explain why?
(a) CH3CH2CH2OH
(b)
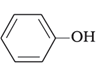
A mixture of NaBr and H2SO4 gives Br2 gas as a product. Molecule (b) will not react with Br2 gas because of the stable molecule that is formed due to resonance stabilization.
2