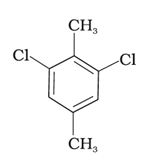
Which of the following compounds will have the highest melting point and why?
(I)
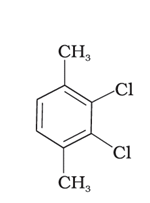
(II)
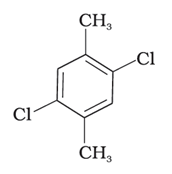
(III)
Compound (II) has both methyl groups as well as chlorine atoms are placed symmetrically at para-positions, due to this, these molecules fit in the crystal lattice better than other isomers, hence it has the highest melting point.
1