Diphenyls are potential threat to the environment. How are these produced from arylhalides?
Diphenyls such as p,p-dichlorodiphenyl trichloroethane (DDT), which have been used as organic pesticides, are a potential threat to the environment because in the early years of its extensive usage, it was shown to have high toxicity towards fish and many insects also developed resistance towards it. The chemical stability of DDT and its fat solubility compounded the problem. In the chemical aspect, DDT is not metabolised very rapidly or solubilized effectively by animals. It is deposited and stored in the fatty tissues. If ingestion continues at a steady rate, DDT builds up within the animal over time, damaging the environment. Diphehyls are produced from arylhalides from two methods, Fittig reaction and Ullman biaryl synthesis.
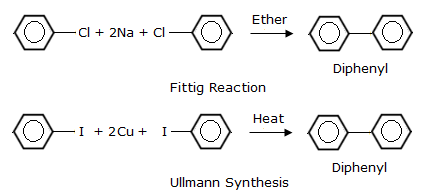
In Fittig reaction, the aryl halides are treated with sodium metal in the presence of dry ether to produce substituted aryl compounds, in this case, biphenyl compound.
In Ullmann synthesis, which is a coupling reaction, aryl halides are heated in the presence of Cu to form biphenyl/diphenyl compounds.