Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution from arylhalides?
(I)
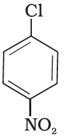
(II)
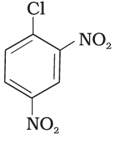
(III)
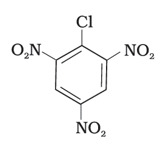
Aryl halides are very less reactive towards nucleophilic substitution because of resonance stabilization. The presence of an –NO2, an electron-withdrawing group at ortho or para position increases the reactivity of the aryl halide towards substitution. The more positions filled with electron withdrawing groups, the more reactive the aryl halide. According to this concept, the decreasing order of reactivity is III > II > I.
1