Match the structures of compounds given in Column I with the classes of compounds given in Column II.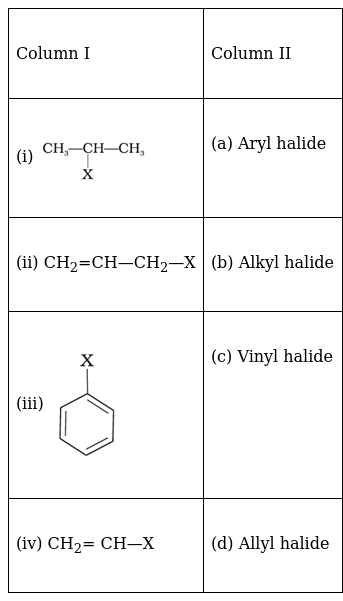
(i) → (b) (ii) → (d) (iii) → (a) (iv) → (c)
The first molecule is an alkyl halide because it contains a halogen atom bound to an sp3 hybridised carbon atom, which is further bound to other alkyl groups.
The second molecule is an allyl halide which are compounds where the halogen atom is attached to a carbon atom is bonded to an sp3 hybridised carbon atom which is attached to a carbon-carbon double bond.
The third molecule is an aryl halide which is a compound where the halogen atom is attached to an aromatic ring carbon atom which is sp2 hybridised.
The fourth molecule is a vinyl halide in which the halogen atom is bound to an sp2 hybridised carbon atom in a carbon-carbon double bond.