Match the reactions given in Column I with the types of reactions given in Column II.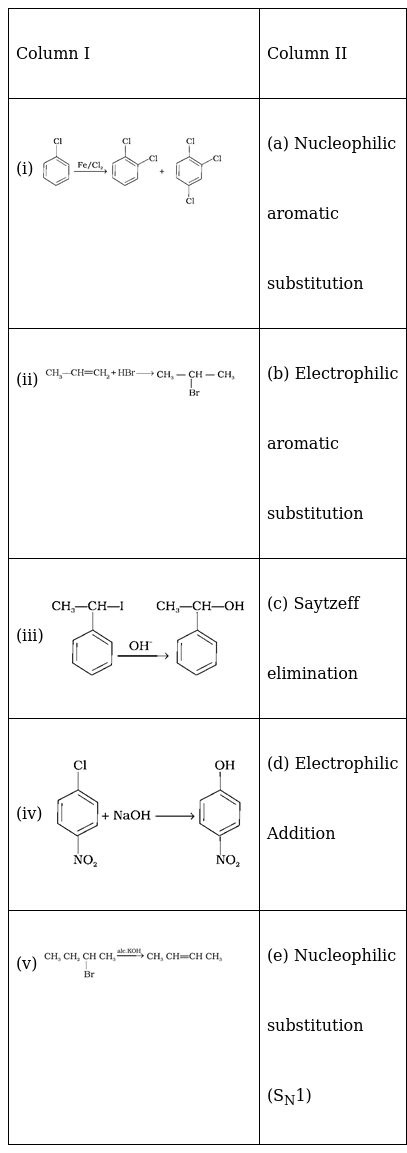
(i) → (b) (ii) → (d) (iii) → (e) (iv) → (a) (v) → (c)
The first reaction is an electrophilic substitution reaction where Cl+ from Cl2 and FeCl3 complex attacks the benzene ring and carry out the substitution.
The second reaction is an electrophilic addition reaction where HBr is added across the double bond following Markownikoff’s rule.
The third reaction is a nucleophilic substitution reaction following the SN1 mechanism where the –I group is replaced by –OH group.
The fourth reaction is a nucleophilic aromatic substitution reaction where the –Cl group is replaced by –OH.
The fifth reaction is a dehydrohalogenation reaction where the alkyl halide follows Saytzeff elimination reaction.