Some alkylhalides undergo substitution whereas some undergo elimination reaction on treatment with bases. Discuss the structural features of alkyl halides with the help of examples which are responsible for this difference.
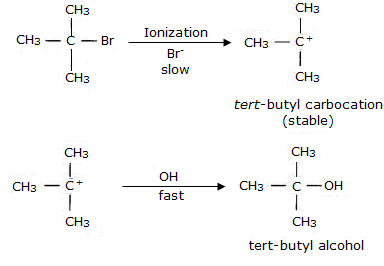
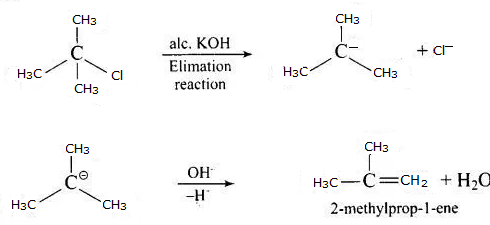
Some alkylhalides undergo substitution or elimination, and this is determined by the structure of the alkyl halide as well as the reagents used in the reaction. To understand the structural features of alkyl halides, their reactivity towards substitution mechanisms can be observed. Primary alkyl halides prefer to undergo substitution reaction by SN2 mechanism which is a one-step reaction involving cleavage of the halide from the carbon atom and simultaneous attachment of the attacking nucleophile. On the other hand, tertiary halides undergo elimination reaction due to the formation of stable carbocation. Tertiary halides also prefer to undergo SN1 reaction which is a two-step process mediated by the formation of a stable carbocation after cleavage of the halide atom. Secondary halides have intermediate reactivity towards both elimination and substitution reactions, depending on the solvent and temperature conditions.
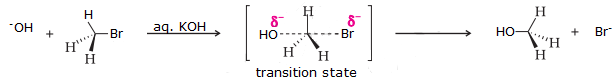
The given reaction is for a primary alkyl halide undergoing SN2 substitution.
The given reaction is for a tertiary alkyl halide undergoing SN1 substitution. The mechanism is determined by the strength of the base used. In case of usage of weak base, aqueous KOH, the reaction undergone is substitution while in case of strong base, alcoholic base, the reaction undergone is elimination.