Addition of water to alkynes occurs in acidic medium and in the presence of Hg2+ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions.
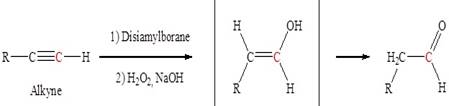
The process of addition of water to ethyne in the presence of H2SO4 and HgSO4 is known as hydration of alkynes.
When hydration of butyne takes place, the product is a ketone.
The OH group gets attached to the secondary carbocation (which is more stable due to the electron giving the property of alkyl group attached to it) after breaking of the triple bond.
The formation of an intermediate enol takes place. The deprotonation of oxygen takes place (i.e. hydrogen of oxygen is removed) to give a more stable final product which is ketone. Thus option (ii) is correct.
Thus, option (i) is wrong as primary carbocation is not stable, option (iii) is a carboxylic acid so it is also wrong and option (iv) is also wrong as the product is a carboxylic acid.
General reaction mechanism: