The correct order of increasing acidic strength is _____________.
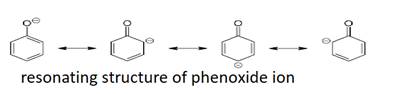
Phenols are more acidic than the alcohols due to to the more stability of phenoxide ion than alkoxide ion due to the resonance of electron pairs of oxygen with the aromatic ring.
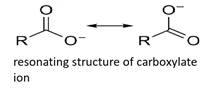
Carboxylic acid is more acidic than phenol as the conjugate base of carboxylic acid, a carboxylate ion, is stabilized by two equivalent resonance structures in which the negative charge is at the more electronegative oxygen atom.
The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom.
Thus, a carboxylate ion is more stable than a phenoxide ion.
When an electron-withdrawing group like chlorine is present on the carboxylic acid its acidity increase due to the fact that the O-H bond gets weaker due to the decrease in the negative charge of an oxygen atom.
Thus, the correct order of acidity in increasing order is:
Alcohols<phenols<acetic acid<choloroacetic acid.