Alkenes 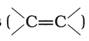 and carbonyl compounds
and carbonyl compounds ![]() , both contain a p bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
, both contain a p bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
In both alkenes and carbonyl compounds, carbon atom is attached to an oxygen atom by a double bond. We know, the electronegativity of oxygen is greater than that of carbon atom. Therefore, oxygen atom in carbonyl compound pull more shared pair of electron towards itself and so, carbon acquires partial positive charge and oxygen acquires partial negative charge in carbonyl compounds. So, carbon in carbonyl atom is attacked by a nucleophile.
Hence, alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions.
1