Carboxylic acids contain carbonyl group but do not show the nucleophilic addition reaction like aldehydes or ketones. Why?
We know, oxygen atom in carbonyl compound pull more shared pair of electron towards itself and so, carbon acquires partial positive charge and oxygen acquires partial negative charge in carbonyl compounds. So, the carbon in carbonyl atom is attacked by a nucleophile.
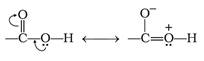
Though carboxylic acids contain carbonyl group they do not show the nucleophilic addition reaction like aldehydes or ketones and this is due to the resonance. The partial positive charge on the carbonyl atom gets reduced because of the resonance in carboxylic acids.
1