Assertion : Formaldehyde is a planar molecule.
Reason : It contains sp2 hybridised carbon atom.
According to Molecular orbital theory (MOT), the carbon in the carbonyl groups (C=O) is actually sp2 hybridized (consisting one s and two p orbitals) and therefore it contributes 3 sp2 orbitals for ∂- bonds (sigma i.e.single bonds) and the 4th valence electron (as carbon has a valency of 4) remains in its (C) one unhybridized partially filled p-orbital.
• This unhybridized p orbital forms a π bond (pi i.e. double bond) by collateral or sidewise overlapping with the partially free p-orbital of the oxygen atom.
• Thus the carbonyl atom of formaldehyde and the other 3 atoms attached to it ( 2 H atoms and one O atoms through sigma bonding) lie in the same plane while the π electron cloud (pi bonds are not actual bonds, they are more like electronic attractions) lies above and below of this planar structure.
• 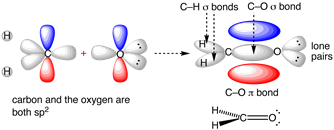
• In addition the lone pairs left on the oxygen atom also lie in the same plane results in a planar structure for formaldehyde (HCHO) with bond angles of almost 120◦(angle for triagonal planar structures).